Our production, processing, and storage facilities are designed to ensure maximum safety, efficiency, and product integrity.
Each area is carefully planned and constructed to allow easy cleaning, maintenance, and to prevent cross-contamination or material mixing.
Operations are conducted in dedicated, appropriately sized spaces, with separate zones and control systems to ensure a clean and controlled workflow.
Raw material, in-process material, and personnel follow separate entry routes to eliminate cross-contact.
Floors, walls, and ceilings feature smooth, easy-to-clean surfaces for enhanced hygiene.
Environmental conditions such as temperature and humidity are continuously monitored and recorded by a BMS (Building Management System) implemented by Kieback&Peter – Germany.

Our cleanroom air systems are engineered for precision and safety. Air cleanliness, volume, temperature, humidity, and pressure are continuously monitored and recorded in full compliance with cGMP standards. The air quality meets Class 100,000 requirements, ensuring a controlled and contamination-free environment.
All HVAC units are designed and manufactured by GEA Klimatechnik GmbH & Co. KG – Austria. The system uses gas heating and water-based Chillers for cooling. Air is recirculated, filtered, and cleaned to prevent both cross-contamination and environmental pollution.
The entire process — from air conditioning to ventilation — is fully automated and monitored through a BMS microprocessor-based controller system, ensuring optimal conditions at every stage.

Our facility is equipped with two dedicated water circulation systems: Sanitary Water and Highly Purified (HP) Water.
Sanitary Water is used exclusively in service areas for non-critical cleaning outside the Clean Rooms.
Highly Purified Water is utilized within manufacturing areas for cleaning both production spaces and equipment.
Designed and implemented to meet cGMP standards, the entire system ensures that water quality complies with both European Pharmacopoeia (Ph. EUR) and United States Pharmacopeia (USP) requirements. Parameters such as temperature, pH, conductivity, total organic carbon, lead, nitrates, and microbiological content are strictly monitored.
This water treatment system plays a vital role in pharmaceutical production, as the purified water either directly contacts or indirectly supports product integrity.
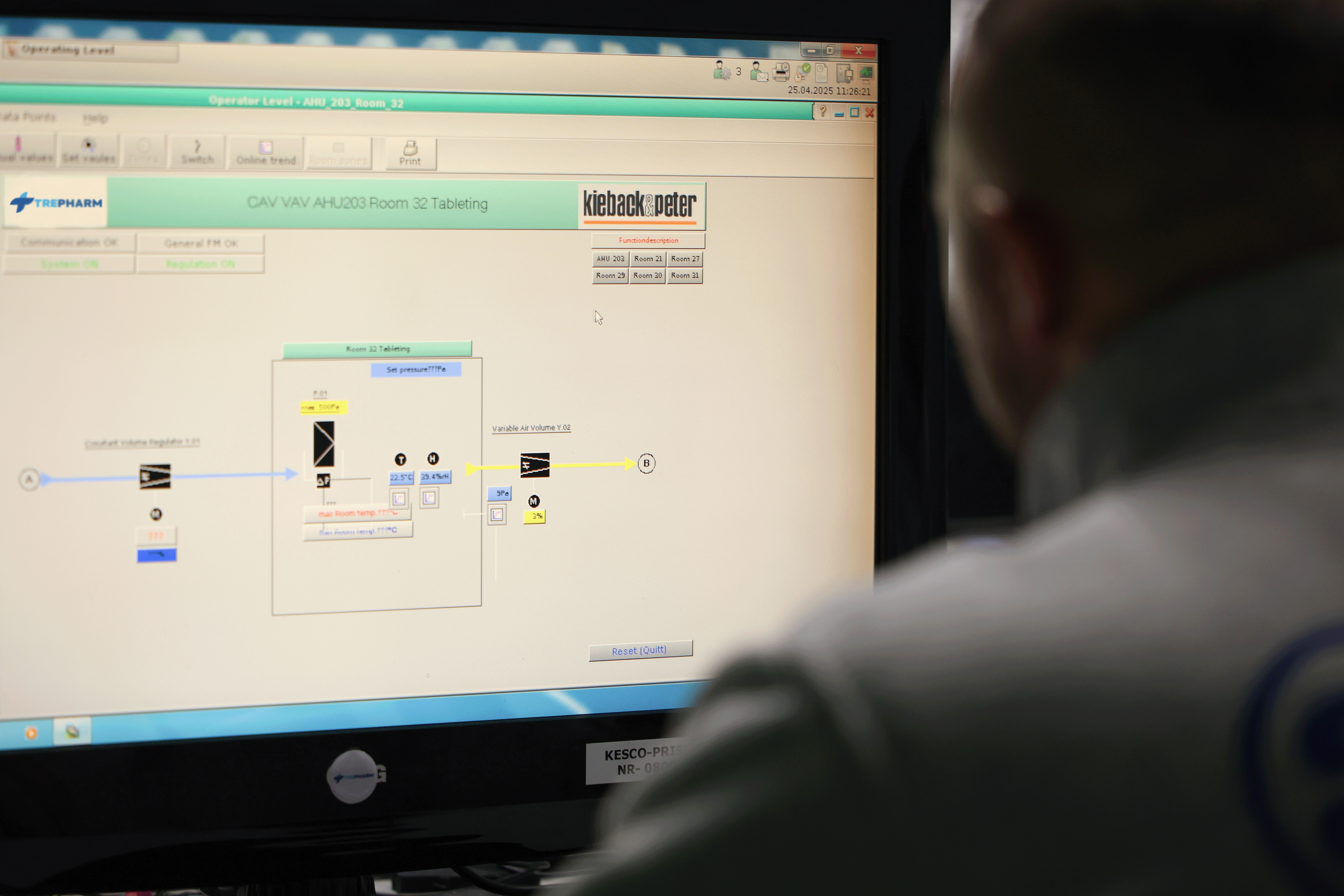
The facility is equipped with an advanced monitoring system that tracks key environmental parameters, including temperature and humidity, to ensure optimal conditions for product integrity. This data is continuously collected and securely stored in the Building Management System (BMS), implemented by Kieback & Peter, Germany. The system offers real-time monitoring, alerting operators to any deviations from predefined thresholds, enabling immediate corrective actions. This proactive approach minimizes the risk of spoilage and ensures that the facility meets regulatory standards. Additionally, the data collected supports long-term performance analysis, helping to optimize energy usage, improve operational efficiency, and maintain consistent environmental conditions across the entire facility.

The facility features a comprehensive Clean Room installation system designed to meet the highest standards of hygiene and safety. This includes cover panels, wall panels, doors, pass boxes, windows, ceilings, laminar flow ceilings, and change parts, all tailored to ensure controlled and sterile environments. The Clean Room systems are installed in full compliance with EN ISO 14644 standards, ensuring that all areas maintain the required levels of cleanliness and air quality for safe and efficient production. These installations are essential for industries where contamination control is critical, such as pharmaceuticals and electronics.

The production buildings are designed with functionality and efficiency in mind, facilitating cleaning, maintenance, and smooth movement of materials and personnel. Each area intended for the production, processing, or storage of pharmaceutical products is carefully constructed and positioned to minimize the risk of contamination and ensure easy cleaning and maintenance.
Specific areas are allocated for different processes, with dedicated control systems in place to prevent material mixing and cross-contamination. Entry routes for raw materials, in-process materials, and personnel are strategically separated to avoid any potential interactions.
The facility features smooth, easy-to-clean flooring, walls, and ceilings. Floors in production areas are constructed with epokal cement, topped with an epoxy layer, while non-production areas, such as administration and archive spaces, are covered with tiles.
Electrical systems throughout the facility are designed and installed in full compliance with local laws and regulations. The lighting system, installed by “GEA,” ensures optimal visibility in all areas and meets the EU CE 73/23/EEC directive, amended with directive 93/68/EEC, for safety and efficiency.

Quality assurance at “Trepharm” is a comprehensive system designed to prevent failures and ensure the safety, quality, and compliance of our pharmaceutical products. It encompasses a wide range of safety measures, quality standards, and regulations to ensure that defective products do not reach advanced stages of the distribution chain.
The quality management system at “Trepharm” integrates WHO directives, laws, and regulations to guide the production process. This dynamic system is continuously evolving to meet the needs of our clients, ensuring product safety, potency, quality, purity, uniformity, credibility, and stability. It emphasizes ongoing development and improvement, aiming to exceed expectations at every stage of production.
Quality principles are applied throughout all departments, including maintenance, engineering, production, and quality assurance. These principles are reflected in internal procedures and systems used for facility planning, validation processes, and analysis methods, all in alignment with GMP standards.
The Quality Control Laboratory is a dedicated area, constructed according to GLP standards, ensuring reliable and valid data for assessing the quality and safety of pharmaceutical products. “Trepharm” has designed the laboratory as an independent unit with the necessary resources to operate as a reference laboratory for the region, strengthening its role in ensuring the highest levels of product quality.

The quality management system at TrePharm is meticulously established, documented, and maintained in accordance with current Good Manufacturing Practices (GMP) regulations in the EU, as well as international standards such as ISO 9001:2015, ISO 14001:2015, and ISO 17025:2018.
Quality Control plays a key role in GMP by overseeing the handling of sampling, specifications, and testing, ensuring that all necessary and appropriate tests are conducted before materials, intermediates, and finished products are released for use. This rigorous process ensures that only products meeting the highest quality standards are approved for distribution.
A Qualified Person (QP), with the qualifications and expertise outlined in Article 49 of Directive 2001/83/EC, is responsible for releasing medicinal products into the market. The QP ensures that each batch is manufactured and controlled according to the law, GMP standards, and the terms outlined in the Marketing Authorization (MA).
To maintain product quality, all suppliers of raw and packaging materials are qualified. Manufacturers and suppliers of goods and services relevant to quality are evaluated under the responsibility of quality assurance, in collaboration with affected departments, to ensure compliance with specifications and contracts.
Regular Product Quality Reviews (PQR) are conducted for all pharmaceuticals manufactured, tested, and released, ensuring continuous quality improvement. The documentation system forms a crucial part of the quality management system, ensuring that all records are accurate, complete, and compliant with GMP requirements. Controls are in place to maintain the integrity, availability, and legibility of all documentation.